The LINX System Procedure
The LINX System is a proprietary technology that was designed as a long-lasting solution to gastroesophageal reflux disease (GERD) which is more commonly known as acid reflux. GERD is a chronic problem that plagues millions: an estimated 18-28% of North Americans suffer from the condition. Many physicians treat acid reflux with medications that suppress stomach acid but this merely addresses the symptoms and does nothing to address and correct the cause of the problem.
What is GERD?
GERD is a condition in which stomach contents flow backwards from the stomach into the esophagus. This occurs when the lower esophageal sphincter (LES), the valve where the esophagus meets the stomach, is weakened and no longer works properly. The esophagus does not tolerate this backflow and becomes irritated and swollen by stomach acid. This causes sore throat, hoarseness, coughing, trouble swallowing, heartburn, and chest pain, among other troubling symptoms. If left untreated, gastroesophageal reflux disease can lead to more serious conditions such as esophagitis, Barrett’s esophagus, and even cancer.
How Does The LINX® System Prevent Reflux?
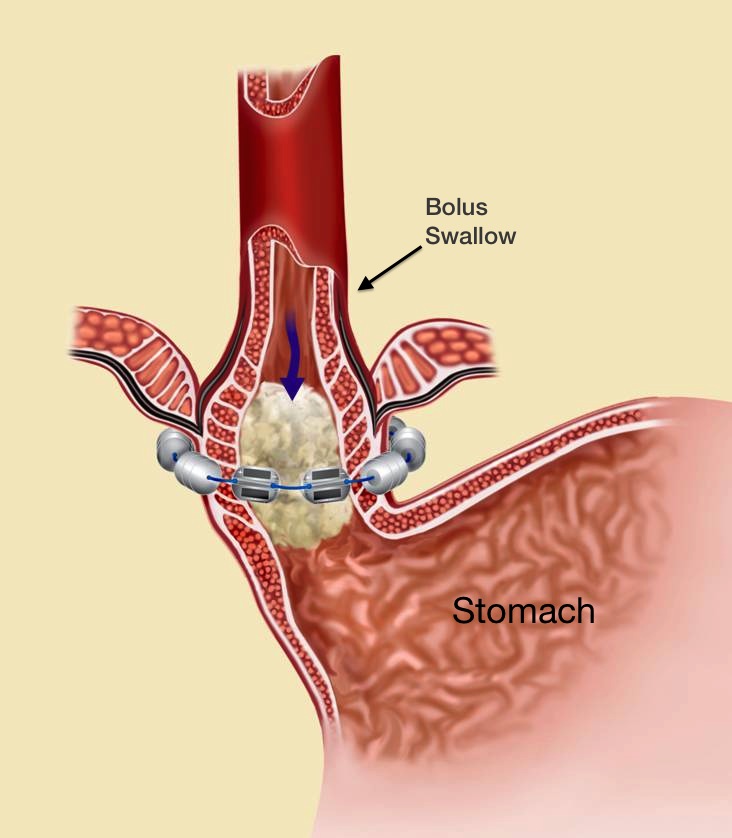
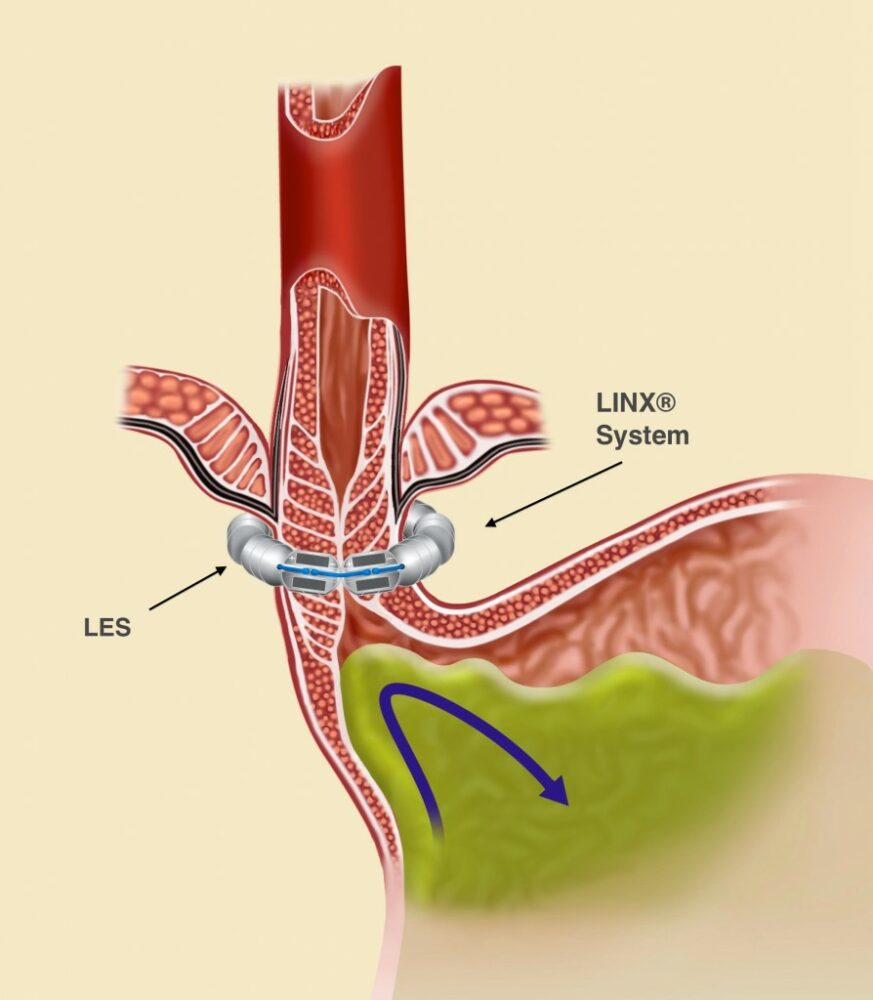
The LINX® System works to stop acid reflux through its unique proprietary technology: a flexible band of magnets encased in titanium beads, then strung together on titanium wire. The LINX System works with the patient’s own body to prevent GERD by keeping the LES closed when it should be closed and allowing it to open when needed.
How Does the LINX Procedure Work?
The attraction between the LINX’s System’s magnetic beads keeps the weakened lower esophageal sphincter (LES) closed most of the time, preventing the backwards flow of acid into the esophagus. When a patient who has had the LINX surgery eats or drinks, the force exerted by the patient’s swallowing reflex temporarily breaks the magnetic bond. (See Figure 1.) This allows food and drink to enter the stomach. After swallowing, magnetic attraction re-closes the LES (Figure 2) and holds it closed, preventing reflux.
During the LINX procedure, your surgeon will put the LINX System in place via laparoscopy, a minimally invasive surgical technique. The procedure is done with general anesthesia and usually takes less than one hour. Once in place, the LINX System begins working right away. Patients who have the LINX procedure are able to eat and drink the same day and return to normal activities within a couple of days. As always, patients should consult their surgeon for their own post LINX surgery instructions.
Who is Eligible for LINX Surgery?
Otherwise healthy adults who suffer with acid reflux disease and have not obtained relief of their symptoms through treatment with proton pump inhibitors (PPI) should be eligible for LINX surgery, but a consultation with a physician is necessary to confirm eligibility. Women who are pregnant, nursing, or who may become pregnant should wait until after pregnancy and lactation is completed. Contact the office of Dr. Preeti Malladi to set up a LINX procedure consultation.
LINX Procedure Recovery
The LINX surgery is performed under general anesthesia and takes approximately one hour. You will be able to return home after a few hours of recovery. You will be given medication to control any pain that you experience from your surgery. You will have five to six small incisions from your minimally invasive surgery and you will experience some soreness during the healing process. You should be sure to move about each day to avoid developing thrombosis (blood clots). Avoid lifting anything heavy (20 lb.s or more) for six weeks after surgery.
You will be on a soft diet for the first few days after your procedure. You gradually reintroduce regular foods into your diet. Be sure to drink plenty of liquids. Your doctor will go over dietary restrictions and your medication list with you. Be sure to follow your surgeon’s post operative instructions carefully. You will probably want to take several days off from work to rest and heal. Most patients are ready to go back to work within 4-10 days of their surgery.